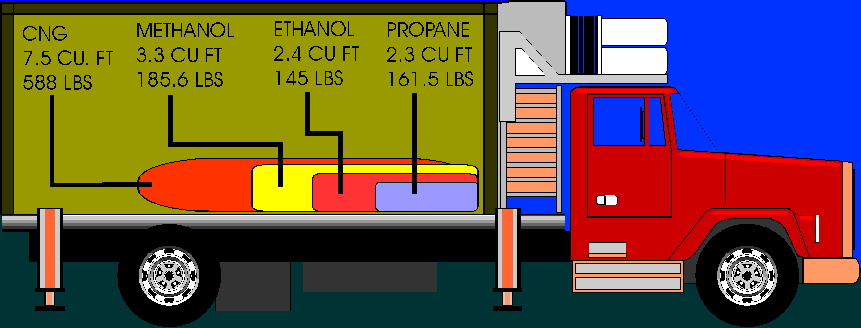
| Physical State |
Gasoline Liquid |
Liquified Petroleum Gas |
Compressed Natural Gas |
Methanol Liquid |
Ethanol Liquid |
| Net Energy Content BTU/lb |
18,700 19,100 |
19,800 | 21,300a | 8,600 | 11,500 |
| Octane Number Range (R + M) ÷ 2 |
87 - 93 | 104b | 120b | 99 | 100 |
| Sulfur Content (W + %) |
0.02 - 0.045 | Negc | Negc | None | None |
Source: Steering A New Course: Transportation, Energy, and the Environment, pages 75, 76.