|
The precise details regarding the twin problems of origin, and migration and accumulation of petroleum have yet to be fully answered. Recent advances in analytical chemistry and geochemistry have advanced the knowledge and understanding, but issues remain to be resolved. The oil pool is an end product to a 5-stage sequence of events: raw materials, accumulation, transformation, migration and geologic time. A better understanding of how accumulation and transformation take place would help clarify the whole process. But the complication is that Petroleums are complex mixtures of many hydrocarbons occurring in homologous series with no two Petroleums exactly alike in composition. This is probably due to variations in primary source materials and subsequent processes during formation such as catalysis, polymerization, pressure and temperature changes. Although the components of petroleum unite to form complex mixtures,
Any theory regarding the origin of petroleum must explain two sets of observations, one geological and the other chemical. Geological observations are that major accumulations:
Traces of indigenous hydrocarbons are also found in igneous and metamorphic rocks and in Chondritic meteorites. Chemical observations are:
Inorganic Hypothesis: There are two theories of origin: Organic (bionic) or Inorganic (abionic). Early theories postulated an inorganic origin when it became apparent that there were widespread deposits of petroleum throughout the world. Dmitri Mendele'ev (1877), a Russian and the father of the periodic table of elements, reasoned that metallic carbides deep within Earth reacted with water at high temperatures to form acetylene (C2H2) which subsequently condensed to form heavier hydrocarbons. This reaction is readily reproduced in the laboratory. Other hypotheses (Berthelot, 1860, Mendele'ev, 1902) were a modification of the acetylene theory. They theorized that the mantle contained iron carbide which would react with percolating water to form methane: FeC2 + 2H2O = CH4 + FeO2
The problem was and still is the lack of evidence for the existence of iron carbide in the mantle. These theories are referred to as the deep-seated terrestrial hypothesis.
Another inorganic hypothesis was suggested by Sokoloff (1890) who proposed a cosmic origin. His theory was one of hydrocarbons precipitated as rain from original nebular matter from which the solar system was formed and then ejected from earth's interior onto surface rocks. This theory and others like it are referred to as the extraterrestrial hypothesis.
20th Century variants and a renewed interest to the inorganic mode of origin by others was caused by two discoveries: Existence of carbonaceous chondrites (meteorites) and the discovery that atmospheres containing methane exists for some celestial bodies such as Saturn, Titan, Jupiter. The only known source for methane is through inorganic reactions. It has been postulated that the original atmosphere of earth contained methane, ammonia, hydrogen, water vapor; add to this photochemical reactions (due to UV radiation) and the result is the creation of an oily, waxy surface layer that may have been host to a variety of developing prebiotic compounds including the precursors of life.
The discovery (Mueller, 1963) of a type of meteorite called carbonaceous chondrites, also led to a renewed interest in an inorganic mechanism for creating organic compounds. Chondritic meteorites contain greater than 6% organic matter (not graphite) and traces of various hydrocarbons including amino acids. The chief support of an inorganic origin is that the hydrocarbons methane, ethane, acetylene, and benzene have repeatedly been made from inorganic sources. For example, congealed magma has been found on the Kola Peninsula in Russia (Petersil'ye, 1962) containing gaseous and liquid hydrocarbons (90% methane, traces of ethane, propane, isobutane). Paraffinic hydrocarbons have also been found in other igneous rocks (Evans, Morton, and Cooper, 1964).
There are problems however, with the inorganic hypotheses. First, there is no direct evidence that will show whether the source of the organic material in the chondritic meteorites is the result of a truly inorganic origin or was in an original parent material which was organically created. Similar reasoning applies to other celestial bodies. Second, there is no field evidence that inorganic processes have occurred in nature, yet there is mounting evidence for an organic origin. And third, there should be large amounts of hydrocarbons emitted from volcanoes, congealed magma, and other igneous rocks if an inorganic origin is the primary methodology for the creation of hydrocarbons. Gaseous hydrocarbons have been recorded (White and Waring, 1963) emanating from volcanoes, with methane (CH4) the most common. Volumes are generally less than 1%, but as high as 15% have been recorded. But the large pools are absent from igneous rocks. Where commercial accumulations do occur, they are in igneous rocks that have intruded into or are overlain by sedimentary materials; in other words, the hydrocarbons probably formed in the sedimentary sequence and migrated into the igneous material (more on this later when we discuss traps).
Conclusion: The are unquestioned instances of indigenous magmatic oil. But the occurrences are rare and the volumes of accumulated oil (pools) are infinitesimally low. Other problematic issues: Commercial accumulations are restricted to sedimentary basins, petroleum seeps and accumulations are absent from igneous and metamorphic rocks, and gas chromatography can fingerprint the organic matter in shales to that found in the adjacent pool. Thus current theory holds that most petroleum is formed by the thermal maturation of organic matter - An Organic Origin generated the vast reserves (pools) of oil and gas.
Organic Hypothesis: There are a number of compelling reasons that support an organic development hypothesis. First and foremost, is the carbon-hydrogen-organic matter connection. Carbon and Hydrogen are the primary constituents of organic material, both plant and animal. Moreover, carbon, hydrogen, and hydrocarbons are continually produced by the life processes of plants and animals. A major breakthrough occurred when it was discovered (Smith, 1952; Smith, 1954; Stevens, 1956; Hunt, 1957; Meinschein, 1959; Erdman, 1961; Kvenvolden, 1964; Silverman, 1965) that hydrocarbons and related compounds occur in many living organisms and are deposited in the sediments with little or no change.
Second were observations dealing with the chemical characteristics of petroleum reservoirs. Nitrogen and porphyrins (chlorophyll derivatives in plants, blood derivatives in animals) are found in all organic matter; they are also found in many petroleums. Presence of porphyrins also mean that anaerobic conditions must have developed early in the formation process because porphyrins are easily and rapidly oxidized and decompose under aerobic conditions. Additionally, low Oxygen content also implies a reducing environment. Thus there is a high probability that petroleum originates within an anaerobic and reducing environment.
Third were observations dealing with the physical characteristics. Nearly all petroleum occurs in sediments that are primarily of marine origin. Petroleum contained in non-marine sediments probably migrated into these areas from marine source materials located nearby. Furthermore, temperatures in the deeper petroleum reservoirs seldom exceed 300oF (141oC) . But temperatures never exceeded 392oF (200oC) where porphyrins are present because they are destroyed above this temperature. Therefore the origin of petroleum is most likely a low-temperature phenomenon.
Finally, time requirements may be less than 1MM years; this is based on more recent oil discoveries in Pliocene sediments.
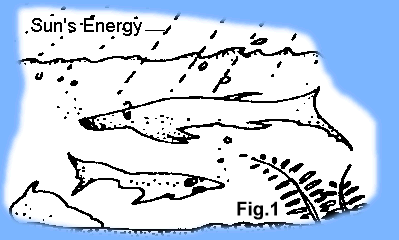
Organic Hypothesis - Summary. The organic theory became the accepted theory about the turn of the century as the oil and gas industry began to fully develop and geologists were exploring for new deposits. Simply stated, the organic theory holds that the carbon and hydrogen necessary for the formation of oil and gas were derived from early marine life forms living on the Earth during the geologic past -- primarily marine plankton. Although plankton are microscopic, the ocean contains so many of them that over 95% of living matter in the ocean is plankton. The Sun's energy provides energy for all living things including plankton and other forms of marine life (Fig.1)
Petroleum End Product = ([Raw Material + Accumulation + Tranformation + Migration] + Geologic Time)
We have spent some time on the two major hypotheses of the origin of petroleum. Now, let's take a closer look at the elements of the formula noted above. In this section we will look at the raw material that eventually ends up as "petroleum." On other pages we will look at how petroleum gets from one place to another (migration) and then forms into large pools (accumulation).
Raw Material: The generalized concept is that petroleum is a natural component of a particular cycle of aqueous sedimentation, usually marine, and requires no abnormal circumstances for its creation such as metamorphism. This generalized concept is reinforced by 3 lines of biochemical evidence:
A major breakthrough occurred when it was discovered (Smith, 1952; Smith, 1954; Stevens, 1956; Hunt, 1958; Meinschein, 1959; Erdman, 1961; Kvenvolden, 1964; Silverman, 1965) that hydrocarbons and related compounds occur in many living organisms and that these same hydrocarbons are deposited in the sediments with little or no change. Virtually all shales and carbonates contain disseminated organic matter of 3 general types:
The primary source materials are fish and microscopic marine life. Some species of fish contain greater than 50% oil and there have been enough fish since Ordovician time to account for ALL known oil deposits in the world. Moreover, fossilized fish are common in sedimentary strata believed to be source rocks. But fish are nektonic animals, they swim about. Upon death, they sink to the sea floor. The fossil record is full of fish accompanied with other benthonic organisms. But the presence of the latter implies oxygenated waters which implies an operational decay process is in effect and scavengers would be present. This would preclude large accumulation of organic matter. And indeed, the fossilized fish are only the skeletal remains. Thus if not fish, what is left?
Microscopic marine life, plankton, are considered to be the primary source of all hydrocarbons. There are two types of plankton: Phytoplankton are the most important and comprise the bulk of the marine plankton. The most abundant volumetrically, are the Diatoms, siliceous unicellular plants. Diatoms contain minute droplets of oil that accumulate in their cellular structure late in the vegetative period. The other type of plankton is Zooplankton. Foraminifera and Radiolaria are the most widely represented fossils in young oil-bearing strata with Copepods being the most numerous. Modern zooplankton also contain minute oil droplets. The reason for the oil droplets is uncertain; they may be formed during the decay process of the animal, or be a food reserve, or may be developed early in their life cycle as a floating mechanism to reduce their density in water. Whatever the reason, the bulk of evidence favors planktonic aquatic organisms, zooplankton and phytoplankton, as the primary source material for the formation of oil and wet gas.
Plankton (Phytoplankton, Zooplankton) create the oil by synthesizing fatty acids. Fatty acids are essential constituents of animal fats and animal and plant oils. The general formula for these acids are CnH2n+2COOH or CnH2nO2 and they form the largest known source of long-chain molecules. This is important because the molecular structure of fatty acids is similar to the molecular structure in crude oil. Paraffin or methane series are straight-chain hydrocarbons having general formula CnH2n+2 while the naphthene series has carbon ring compounds having the general formula CnH2n.
If the raw materials that make up petroleum are marine plankton, how did so much of it gather in large pools around the world? How did the pools get formed? In the next section we will take a look at the migration and accumulation of petroleum.
Sources: "Geology of Oil," Steven Cooperman, Ph.D. "Understanding Petroleum Exploration and Production," National Energy Foundation, Student Activity Guide "The Upstream: A Guide to Petroleum Exploration and Production," Exxon Corporation Informational Brochure
NORTH, F. K., 1985, Petroleum Geology: Allen & Unwin, Inc., Winchester, MA. |
 Where does petroleum come from?
Where does petroleum come from?
 As these early life forms died, their remains were captured by the processes of erosion and sedimentation (Fig 2). Successive layers of organic-rich mud and silt covered preceding layers of organic rich sediments and over time created layers on the sea floor rich in
As these early life forms died, their remains were captured by the processes of erosion and sedimentation (Fig 2). Successive layers of organic-rich mud and silt covered preceding layers of organic rich sediments and over time created layers on the sea floor rich in 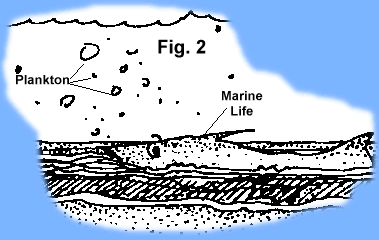 the fossil remains of previous life (Fig. 3). Thermal maturation processes (decay, heat, pressure) slowly converted the organic matter into oil and gas. Add additional geologic time (millions of years) and the organic rich sediments were
the fossil remains of previous life (Fig. 3). Thermal maturation processes (decay, heat, pressure) slowly converted the organic matter into oil and gas. Add additional geologic time (millions of years) and the organic rich sediments were
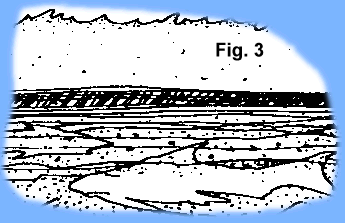 converted into layers of rocks. Add more geologic time and the layers were deformed, buckled, broken, and uplifted; the liquid petroleum flowed upward through porous rock until it became trapped and could flow no further forming
converted into layers of rocks. Add more geologic time and the layers were deformed, buckled, broken, and uplifted; the liquid petroleum flowed upward through porous rock until it became trapped and could flow no further forming
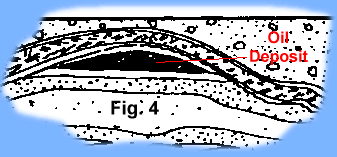 the oil and gas that we explore for at present (Fig. 4). But the chemistry of the hydrocarbons found in the end product (oil, gas) differ somewhat from those we find in living things. Thus changes, transformation, takes place between the deposition of the organic remains and the creation of the end product. The basic formula for the creation of petroleum (oil, gas) is:
the oil and gas that we explore for at present (Fig. 4). But the chemistry of the hydrocarbons found in the end product (oil, gas) differ somewhat from those we find in living things. Thus changes, transformation, takes place between the deposition of the organic remains and the creation of the end product. The basic formula for the creation of petroleum (oil, gas) is: